Herpes simplex virus-1
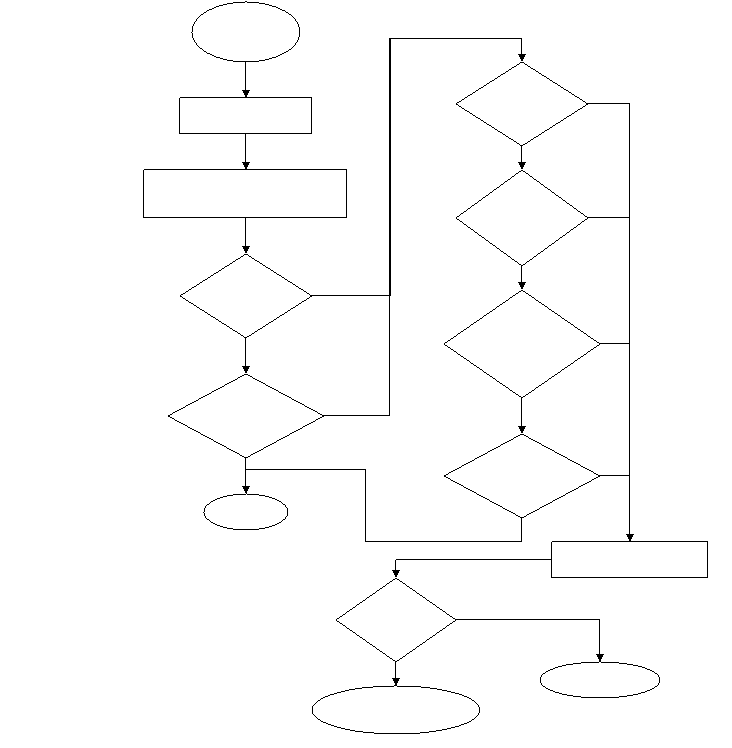
Empirical treatment should be initiated in all suspected cases of herpes simplex encephalitis (HSE) as shown in Figure 1. Herpes simplex virus (HSV) encephalitis is treated with intravenous acyclovir at a dose of 10 mg/kg every 8 hours.95, 96 The length of acyclovir treatment depends on multiple factors. If therapy is initiated because of suspected HSE, but an alternative diagnosis is made, acyclovir should be stopped. If the initial HSV PCR is negative, but the diagnosis of HSE is highly suspected, additional CSF should be obtained for PCR testing 72 hours after symptom onset and CSF for HSV antibodies should be obtained seven days after symptom onset. If HSV PCR is persistently negative, but the MRI demonstrates a lesion of increased signal intensity on T2-weighted and/or FLAIR imaging, the patient should be treated with 21 days of intravenous acyclovir or treated with acyclovir until an alternative diagnosis is made. When HSE is confirmed through PCR or HSV antibodies in the CSF, the patient should receive 21 days of intravenous acyclovir. Though initial studies treated patients for 10 days, one study showed 20% of patients had a positive PCR result after 14 days of treatment.97 No formal studies have compared 10 days to 14 days to 21 days of acyclovir therapy. One study treated patients with a median of 14 days of acyclovir and reported 3 of 36 patients (8%) required a second course of acyclovir for a clinically suspected relapase.98 Another study reported two relapses out of 53 (4%) when the length of acyclovir therapy was 10 days.95 Based on limited data and the relative safety of acyclovir, all patients should receive 21 days of acyclovir if the diagnosis of HSE is confirmed or strongly suspected.
Steroids have been studied as an adjunctive treatment in HSE. Two small case series showed reduced morbidity in patients treated with intravenous steroids at the onset of symptoms.99, 100 Future studies may confirm a benefit of steroid therapy, but currently there is not sufficient evidence to recommend corticosteroid therapy for HSE. Other therapies, including the use of interferons to prevent HSV-mediated neuronal death have been investigated in mice, but not in humans.101, 102
In 5% to 30% of immunocompromised patients, acyclovir-resistant herpes simplex viruses have been isolated. Acyclovir is a purine nucleoside analog that is activated by herpes virus thymidine kinase. The activated acyclovir inhibits herpes DNA polymerase and prevents replication. Mutations in the viral genes encoding for thymidine kinase or DNA polymerase render acyclovir useless. Other thymidine kinase-dependant medications such as valacyclovir, penciclovir and famciclovir are also useless in treating resistant herpesviruses.103 Foscarnet inhibits herpes DNA polymerase independent of thymidine kinase function. Dosed at 60 mg/kg every 8 hours for 21 days, it is effective in treating the majority of acyclovir-resistant herpesviruses, which have a thymidine kinase mutation.103, 104 However, some acyclovir-resistant herpesviruses, which develop resistance based on mutations in DNA polymerase, are also resistant to foscarnet.105 Several articles report successful treatment of mucocutaneous HSV infections with cidofovir.106-109 One study reported that all acyclovir-resistant, foscarnet-resistant strains isolated in vitro were sensitive to cidofovir.110 However, a recent case series reported three patients with cutaneous HSV infections that were unresponsive to cidofovir. One of the three HSV isolates demonstrated in vitro resistance to cidofovir.111 There is no standard dose for the treatment of herpes simplex encephalitis with cidofovir.
No randomized, controlled trials have compared the efficacy of acyclovir to any other viral agent in treatment of varicella-zoster virus (VZV) encephalitis. Traditionally acyclovir has been used because of its relative safety and proven efficacy against HSV. The genetic similarity between VZV and HSV thymidine kinase suggests that therapies against HSV would also be effective against VZV.112 Treatment with acyclovir 10 mg/kg IV three times per day for 14 to 21 days is recommended. Additionally, the use of steroids has been recommended to treat the inflammation associated with small or large-vessel vasculitis.113 The addition of high-dose intravenous corticosteroids should be considered in patients that do not have a contraindication to the use of steroids.
Ganciclovir has been the mainstay of treatment for all cytomegalovirus (CMV) related diseases since its approval in 1988.114 The recommended dose of intravenous ganciclovir for CMV encephalitis is 5 mg/kg IV every 12 hours for two weeks followed by maintenance therapy of 5 mg/kg/day IV.115, 116 Recently, oral valganciclovir became available, offering 10 times the bioavailability of oral ganciclovir117 and similar bioavailability to intravenous ganciclovir.118, 119 No studies report a benefit of oral valganciclovir over intravenous ganciclovir in CMV encephalitis. Cidofovir and foscarnet are also used in the treatment of CMV-encephalitis.114 The recommended dose of foscarnet for CMV encephalitis or retinitis is 180 mg/kg/day (either 60 mg/kg every 8 hours or 90 mg/kg every 12 hours) intravenously for two weeks followed by 60-120 mg/kg daily.120, 121 The use of foscarnet as an adjunct to ganciclovir in CMV encephalitis has been advocated,115, 116 and is supported by one in vitro study122 and one in vivo study treating CMV retinitis.121 The major limitation of the combination therapy is toxicity. However, a study comparing full dose ganciclovir to half-dose ganciclovir plus half-dose foscarnet showed no superiority of combination therapy.123
The optimal duration of therapy for any of the anti-CMV medications remains unclear. None of the agents completely eradicates the infection, so maintenance therapy is often required long-term for viral suppression. If a patient had CMV encephalitis while in a temporary immunocompromised state and there is reconstitution of the immune system, maintenance therapy could be stopped. However, the majority of patients with CMV encephalitis have an underlying chronic immunodeficiency that may require life-long anti-CMV therapy to prevent recurrences. Our recommendation is to treat with two weeks of full dose ganciclovir (5 mg/kg every 12 hours IV) followed by maintenance therapy (5 mg/kg daily IV). Patients that relapse, or fail to improve after two weeks, should receive full-dose foscarnet therapy for two weeks (60 mg/kg every 8 hours IV) followed by foscarnet maintenance therapy (60-120 mg/kg daily IV) indefinitely. Alternative regimens include the combination of ganciclovir and foscarnet (in full doses as outlined above) or cidofovir. If the combination of ganciclovir and foscarnet causes significant adverse effects, one of the agents can be stopped and the other continued. Another option is to use cidofovir 5 mg/kg weekly IV for 2 weeks, then 5 mg/kg every 2 weeks IV for maintenance. Patients should be pretreated with probenecid 2 grams orally 3 hours before the cidofovir, then an additional 1 gram two and eight hours after the cidofovir.
Human herpes virus type 6 and 7
Although no clinical trials have established the efficacy of any one antiviral agent in the treatment of HHV-6 encephalitis, some small series indicate successful treatment with either ganciclovir or foscarnet.124-127 The course of treatment with ganciclovir or foscarnet has not been established, but because the in vitro response to antiviral medications resembles that of CMV, treatment of HHV-6 encephalitis should be the same as the treatment for CMV encephalitis.128 Treatment with donor leukocyte infusion has been described, but remains an unproven therapy.124 Because of the similarity of HHV-7 with HHV-6, treatment of HHV-7 encephalitis should be the same as treatment of HHV-6 encephalitis.
No therapy has proven to decrease morbidity associated with Epstein-Barr virus (EBV) encephalitis. Although studies do show decreased shedding of virus with acyclovir therapy, there is no statistically significant benefit to acyclovir therapy in infectious mononucleosis.129 There are anecdotal reports of the benefit of acyclovir and ganciclovir in the treatment of EBV encephalitis, but definitive treatment has not been established by clinical trials. Corticosteroids have been shown to improve EBV-related acute disseminated encephalomyelitis, but not EBV encephalitis.130 Because of the generally favorable outcomes in EBV encephalitis, no specific antiviral or immunomodulating therapies are recommended.
Figure 1. Treatment algorithm for herpes encephalitis.

*fever, headache, and either an altered level of consciousness, new-onset seizures, or a focal neurological deficit.